
What Is Alzheimer's Disease?

Alzheimer’s Disease (AD for short) is a progressive brain disorder that slowly destroys memory and thinking skills and, eventually, the ability to carry out the simplest of tasks.
In most people with the disease, those with the late-onset type, symptoms first appear in their mid-60s. Alzheimer’s Disease is the most common cause of dementia among older adults.
The disease is named after Dr. Alzheimer, who was a German psychiatrist and neuro-pathologist. In 1906, Dr. Alzheimer noticed changes in the brain tissue of a woman who had died of an unusual mental illness. Her symptoms included memory loss, language problems, and unpredictable behavior. After she died, he examined her brain and found many abnormal clumps (now called amyloid plaques) and tangled bundles of fibers (now called tau tangles).
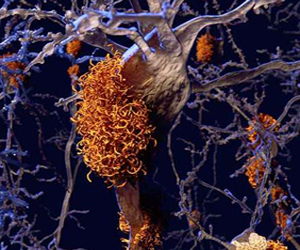
The plaques and tangles in the brain are still considered some of the main features of Alzheimer’s Disease. See the "orange amyloid plaques" from a computer generated brain image to the left. Another AD feature is the loss of connections between nerve cells (neurons) in the brain. Neurons transmit messages between different parts of the brain, and from the brain to muscles and organs in the body.
Many other complex brain changes are thought to play a role in Alzheimer’s, too. AD causes probably include a combination of genetic, environmental, and lifestyle factors. The importance of any one of these factors in increasing or decreasing the risk of developing Alzheimer’s differs from person to person.
Estimates vary, but experts suggest that more than 5.5 million Americans may have Alzheimer’s. AD is currently ranked as the sixth leading overall cause of death in the United States, but recent estimates indicate that the disorder may rank third, just behind heart disease and cancer, as a cause of death for people older than 65.
Alzheimer's Signs, Symptoms, and Stages
Memory problems are typically one of the first signs of cognitive impairment related to Alzheimer’s Disease. Some people with memory problems have a condition called mild cognitive impairment (MCI). In MCI, people have more memory problems than normal for their age, but their symptoms do not interfere with their everyday lives. Movement difficulties and problems with the sense of smell have also been linked to MCI. Older people with MCI are at greater risk for developing Alzheimer's, but not all of them do. Some may even go back to normal cognition.
The first symptoms of AD (Alzheimer's Disease) vary from person to person. For many, a decline in non-memory aspects of cognition, such as word-finding, vision/spatial issues, and impaired reasoning or judgment, may signal the very early stages of AD. Researchers are studying biomarkers (biological signs of the disease found in brain images, brain/spinal fluid, and blood) to see if they can detect early changes in the brains of people with MCI and in cognitively normal people who may be at greater risk for Alzheimer’s. Studies indicate that such early detection may be possible, but more research is needed before these techniques can be relied upon to diagnose Alzheimer's Disease in everyday medical practice.
Early-Onset of Alzheimer's
Early-onset AD occurs between a person’s 30s to mid-60s and represents less than 10 percent of all people with Alzheimer’s. Some cases are caused by an inherited "change" in one of three critical genes (explained below), resulting in a type known as early-onset "Familial Alzheimer’s Disease, or FAD. For other cases of early-onset Alzheimer’s, research suggests there may be a genetic component related to factors other than the three critical genes.
Late-Onset of Alzheimer's
Most people with Alzheimer's (90 %) have the late-onset form of the disease, in which symptoms become apparent in the mid-60s and later. The causes of late-onset Alzheimer's are not yet completely understood, but they likely include a combination of genetic, environmental, and lifestyle factors that affect a person's risk for developing the disease.
Mild Alzheimer’s Disease
As AD progresses, people experience greater memory loss and other cognitive difficulties. Problems can include wandering and getting lost, trouble handling money and paying bills, repeating questions, taking longer to complete normal daily tasks, and personality and behavior changes. People are often diagnosed with mild cognitive impairment (MCI) in this stage. Damage initially appears to take place in the hippocampus, the part of the brain essential in forming memories. As neurons (the connectors) die, additional parts of the brain are affected.
Moderate Alzheimer’s Disease
In this stage, damage occurs in areas of the brain that control language, reasoning, sensory processing, and conscious thought. Memory loss and confusion grow worse, and people begin to have problems recognizing family and friends. They may be unable to learn new things, carry out multistep tasks such as getting dressed, or cope with new situations. In addition, people at this stage may have hallucinations, delusions, paranoia and may behave impulsively.
Severe Alzheimer’s Disease
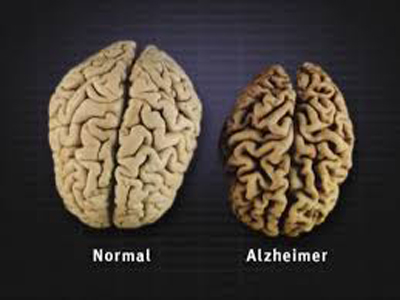
Ultimately, plaques and tangles have spread throughout the brain. The damage is significant and the brain tissue has shrunk considerably (see the above photo). People with severe Alzheimer’s cannot communicate and are completely dependent on others for their care. Near the end, the person may be in bed most or all of the time as the body shuts down. Top
Genes Are Important But Not Definitive
The apolipo-protein E (APOE) gene is very involved in Alzheimer’s Disease. Found on chromosome 19, there are three versions of the APOE gene: APOE2, APOE3 and APOE4 (E2, E3 and E4 for short; note that there is no E1 gene version.). See the artist's conception of an APOE gene to the left. Each type is called an "allele" (pronounced all·lee'l). Every person has two copies of the APOE gene (one from each parent) and the combination determines the person's APOE "genotype" i.e. one of the six possible APOE combinations: E2/E2, E2/E3, E2/E4, E3/E3, E3/E4, or E4/E4.
The three human alleles (E2, E3, E4) arose after the primate-human split around 7.5 million years ago. These alleles are the by-product of mutations which led to changes in functionality. The first allele to emerge was the E4. Besides Alzheimer's, other diseases have been linked to the E4 gene, including heart disease, and gallbladder stones.
A short summary of the APOE alleles follows:
-
APO E2 gene is relatively rare and people with only one E2 gene have a reduced risk of Alzheimer's Disease. The chance of people with two E2 genes developing AD is almost zero. (8.4% of the worldwide population has at least one of this gene).
-
APO E3, the most common allele, is believed to play a neutral role in the disease - neither decreasing nor increasing the AD risk. The chance of people with two E3 genes developing AD is about 9%. (77.9% of the worldwide population has one of this gene).
-
APO E4 increases the risk for AD and is also associated with an earlier age of disease onset. Only people with one or two of the E4 genes need worry about the risk of AD. (13.7% of the worldwide population has at least one of this gene).
-
Most people want a sense of their lifetime AD risk. A good research study looked at 7,351 AD cases and 10,132 non-AD controls from 7 different countries and reported: "At the age of 85 the long term risk of AD, without any reference to APOE genotype, was 11% in males and 14% in females. At the same age of 85, the risk ranged from 51% for APOE 4/4 male carriers to 60% for APOE 4/4 female carriers; and from 23% for APOE 3/4 male carriers to 30% for APOE 3/4 female carriers."
It is very important to note that people with the APOE E4 allele inherit an" increased risk" of developing AD, not the disease itself. Also, some people with "no APOE E4" may develop Alzheimer’s.
One can get their saliva tested at a reasonable price for the three APOE gene types here. Top
Alzheimer's Affects Women More Than Men

Two-thirds of Alzheimer's cases in the United States are women, and it's not just because women live longer, experts say. In the United Kingdom and Australia, dementia is the number one killer of women, knocking heart disease off the top spot. At the Alzheimer's Association International Conference in Los Angeles in July, 2019, scientists offered evidence that the disease may spread differently in the brains of women than in men.
Roberta Brinton, a University of Southern California professor who studies gender differences said, “It is true that age is the greatest risk factor for developing Alzheimer’s disease.” She went on to say, “on average, women live four or five years longer than men, but we know that Alzheimer’s is a disease that starts 20 years before the diagnosis.”
Katherine Lin of Duke University studied 400 people with mild cognitive impairment (MCI). They used an 11-part test that’s routine for diagnosing memory loss and Alzheimer’s. Women, they found, declined at a rate of more than two points a year on the test, compared to men who declined at a rate of just over one point a year.
New research suggests that protein tangles found within neurons and linked to Alzheimer’s disease might spread differently in women’s brains than men’s. The study, which also was presented at the Conference in Los Angeles by researchers from Vanderbilt University, used scans from a method called positron emission tomography. That allowed them to look at the way clumps of a protein called "tau" were spread in the brains of 123 men and 178 women without cognitive problems, as well as 101 men and 60 women with mild cognitive problems although not yet diagnosed with Alzheimer’s disease.
Cognitively normal older people often have small amounts of tau in certain areas of their brain. From their data the Vanderbilt team build maps showing which areas of the brain had similar signals relating to tau. This suggested that they were somehow connected. The team said the results are that the maps from women look different than ones from men. This suggests that tau might be able to spread more rapidly across the female brain.
Social factors might also play a role in why more women develop the disease. Researchers at the University of California Los Angeles reported that their work with more than 6,300 women born between 1935 and 1956 showed that memory decline in older age was faster among those who had not worked. Looking at memory in the women as they aged from 60 to 70, the decline in performance was 61% faster among married mothers who had never worked, compared with married mothers who had worked until middle age.
Some other risk factors for the disease affect women more than men. For example, more women develop depression and a depressed mood has been linked to the onset of Alzheimer’s. Other risk factors affect only women, such as surgical menopause and pregnancy complications like pre-eclampsia, both of which have been linked to cognitive decline in later life.
There are quite a few theories as to why Alzheimer's affects women more than men, but the bottom line is that at this time we really do not know the "technical answer". Top
The Role Of The Hippocampus
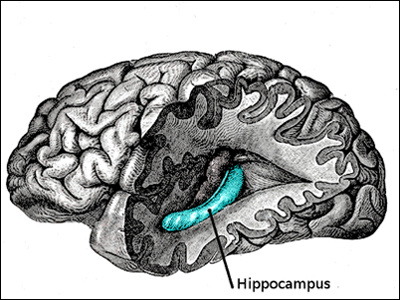
The hippocampus is our "brain's memory center". In the picture of the human brain to the left, the front lobe is at the left and the rear lobe to the right. The temporal and parietal lobes have been removed to reveal the inner location of the hippocampus. The hippocampus is located in the "subcortex" or inner section of the the brain. (The cortex is the large outer section of the brain going from ear to ear and front to back, pictured in grey to the left.)
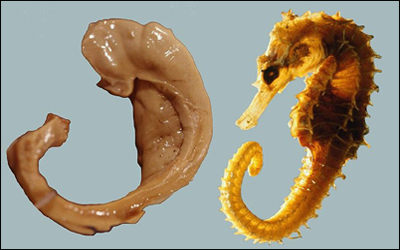
The hippocampus is so-named for its resemblance to the sea horse, pictured to the left below. (In the Greek language, "hippos" means a horse and "kampos" means sea monster.) Humans and other mammals have two hippocampi, one on each side of the brain above the ears.
What Hippocampi Do
Hippocampi play important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus helps us develop new memories. It is the "gateway" for new memories, as memories need to first pass through the hippocampus before being stored in long-term memory banks. Research has shown that the hippocampus is important not only for the formation of new memories, but also for the retrieval of old memories. (Interesting, the hippocampus on the left side of the brain often has a greater function in memory and language than the one on the right side.)
Alzheimer's Effect
Alzheimer’s disease results in progressive loss of tissue throughout the brain. In particular, the hippocampus tends to show the most rapid loss of tissue earliest in the course of the disease. The progressive shrinkage of the hippocampus is responsible for mild cognitive impairment (MCI), the short-term memory loss that is the hallmark symptom of Alzheimer’s. A study found that the volume and ratio of the hippocampus was reduced on average by 25 percent in Alzheimer’s disease.
Can the Hippocampus Be Prevented From Shrinking?
The plasticity (a term for the brain's ability to grow and change over time) of the hippocampus has been demonstrated repeatedly in research. Research has found that although the hippocampus tends to atrophy as we age, both physical exercise and cognitive stimulation (mental exercise) can slow that shrinkage, and.... with a determined approach, even reverse it.
For a good description of how Alzheimer's Disease affects other parts of the brain, click here.
Rescue Your Memory From Alzheimer's - Insulin Resistance

The major hallmarks of Alzheimer’s Disease - amyloid plaques, neuro-fibrillary tangles, and brain cell atrophy - can all be explained by "insulin resistance". A staggering 80% of people with Alzheimer’s Disease have insulin resistance. The connection between insulin resistance and Alzheimer’s Disease is now so firmly established that some scientists have started referring to Alzheimer’s Disease as “Type 3 Diabetes”.
This does not mean that diabetes causes Alzheimer’s Disease - AD can strike even if you don’t have diabetes. It’s more accurate to think of it this way: Insulin resistance of the body is type 2 diabetes; insulin resistance of the brain is type 3 diabetes. They are two separate diseases caused by the same underlying problem: insulin resistance.
Insulin is a powerful hormone that orchestrates how cells access and process vital nutrients, including glucose (sugar). One of insulin’s responsibilities is to unlock cells so they can absorb glucose from the bloodstream. When you eat something sweet that causes your blood sugar to spike, the pancreas releases insulin to usher the excess glucose out of the bloodstream and into cells.
If your blood sugar and insulin spike too high too often, cells will try to protect themselves from overexposure to insulin’s powerful effects by toning down their response to insulin - they become “insulin resistant.” In an effort to overcome this resistance, the pancreas releases even more insulin into the blood to try to keep glucose moving into cells. The more insulin levels rise, the more insulin resistant cells become. Over time, this vicious cycle can lead to persistently elevated blood glucose levels (high A1C readings).
Hippocampal cells require so much energy to do their work that they often need extra boosts of glucose. While insulin is not required to let a normal amount of glucose into the hippocampus, special glucose surges do require insulin. This explains why declining memory is one of the earliest signs of Alzheimer’s. Without adequate insulin, the vulnerable hippocampus struggles to record new memories, and over time begins to shrivel up. By the time a person notices symptoms of Mild Cognitive Impairment, the hippocampus has already shrunk by more than 10%.
The brain sugar processing problem caused by insulin resistance is called “glucose hypometabolism.” This simply means that brain cells don’t have enough insulin to burn glucose at full capacity. The more insulin resistant you become, the more sluggish your brain glucose metabolism becomes. Brain glucose metabolism can be reduced by as much as 25% long before any memory problems become obvious.
The good news is that insulin resistance is a major risk factor for Alzheimer’s Disease that you "CAN" do something about. Eating too many of the wrong carbohydrates too often is what causes blood sugar and insulin levels to rise, placing us at high risk for insulin resistance and Alzheimer’s Disease. Our bodies have evolved to handle whole food sources of carbohydrates, like apples and sweet potatoes, but they simply aren’t equipped to cope with modern refined carbohydrates like flour and sugar.
Here are three steps you can take to minimize your risk for Alzheimer’s Disease:
- Find out how insulin resistant you are. Your A1C should be less than 5.7. The AIC test measures your average blood sugar over the previous two to three months.
- Avoid refined carbohydrates like the plague (i.e. bagels, juice boxes, granola bars, anything with lots of sugar). If you have insulin resistance, an A1C of 5.7 or more, reduce your total carbohydrate intake to less than 40 grams per day.
- Exercise for an hour a day, 5 days a week. Exercise is key to improving memory as it increases the blood flow of oxygen and other goodies to the brain.
You can wield tremendous power over insulin resistance simply by changing the way you eat. Tests for insulin resistance respond surprisingly quickly to dietary changes. Many people see dramatic improvements in their blood sugar and insulin levels within a few weeks. A study showed that a low carbohydrate diet (40 grams or less a day) improved memory in people with Mild Cognitive Impairment in only six weeks. Top
Rescue Your Memory From Alzheimer's - The MIND Diet

The MIND diet, which stands for "Mediterranean-DASH Intervention for Neuro degenerative Delay," was developed by Martha Clare Morris, a nutritional epidemiologist at Rush University Medical Center, through a study funded by the National Institute on Aging. The MIND diet takes two proven diets, the DASH and Mediterranean diets, and zeroes in on the foods in each that specifically deter Alzheimer's. The MIND diet was published online in February, 2015. And while more study is still needed to better understand the long-term impact of the diet, her team’s second paper on the MIND diet notes that it’s superior to the DASH and Mediterranean diets for preventing cognitive decline.
Morris' team followed the food intake of 923 Chicago-area seniors. Over four and a half years, 144 participants (16%) developed Alzheimer's disease. The longer people had followed the MIND diet patterns, the less risk they appeared to have. Even people who made "modest" changes to their diets – who would not have fit the criteria for the DASH or Mediterranean diets – had less risk of developing Alzheimer's. The study found the MIND diet lowered Alzheimer's risk by about 35 percent for people who followed it moderately well and up to 53 percent for those who adhered to it rigorously.
The MIND diet recommends two or more servings of berries and at least six servings of green leafy vegetables per week, eating nuts throughout the week, beans nearly every other day, three servings of whole grains per day, at least one fish meal and two poultry meals per week and one glass of wine per day.
The scientists also looked for five unhealthy food groups. This does not mean one can never consume any of the five bad food groups. Individuals can still follow the MIND diet if they reduce consumption of the brain-damaging foods.
The five brain-damaging foods to reduce (or avoid):
- Pastries and sweets to under five servings per week
- Red meat to under four servings per week
- Cheese to under one serving per week
- Butter or margarine to under one tablespoon per day
- Fried/fast foods to under one serving per week
Much more information on the MIND diet can be found here.
Rescue Your Memory From Alzheimer's - Keep Your Mouth Clean
Of the 200 or so drug studies which have been aimed at plaques for treating Alzheimer's Disease 99.6% have not been successful. A few have delayed the disease somewhat, but none have been able to stop it or cure it.
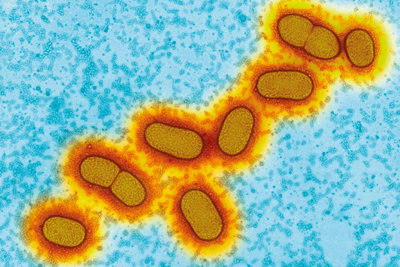
Therefore, researchers have begun to look at other possibilities. Bacteria is near the top of the list of other possible AD causes. A study released in January, 2019 showed that harmful bacteria were found in the brains of 54 people who had Alzheimer’s when they were alive. Porphyromonas Gingivalis (P. Gingivalis for short) is involved in gum disease and is a known risk factor for Alzheimer’s. See the illustration of P. Gingivalis to the left.
Two toxic enzymes that are secreted by the P. Gingivalis bacteria to "feed on human tissue" were found in the AD brain samples - 96% for one enzyme and 91% for the second. The samples were taken from the hippocampus (the brain area critical to memory).
When P. Gingivalis gum disease was inserted into mice in a controlled test, it led to brain infection, amyloid production, tangles of tau protein and neural damage in the regions and nerves normally affected by Alzheimer’s. This suggests causation, said Casey Lynch CEO of Cortexyme, a pharmaceutical firm in San Francisco. However, they recognize that future studies need to be in humans to be convincing.
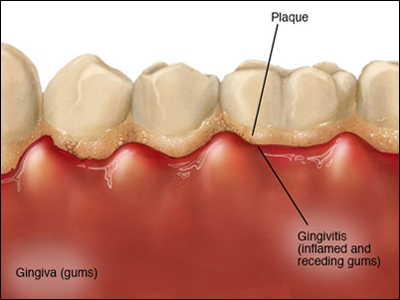
Your mouth normally hosts a diverse and relatively stable community of bacteria, but when dental plaque builds under the edge of your teeth, it can form inflamed pockets in which P.Gingivalis can thrive and release toxins. See the illustration to the left. This inflammation can lead to chronic periodontitis and tooth loss, and some studies have shown that people with fewer teeth are more likely to have dementia. The inflammation and toxins caused by P. Gingivalis damage the lining of your mouth, which may make it possible for oral bacteria to enter the bloodstream and then invade other organs.
For how P. Gingivalis might cause dementia after it arrives in the brain, there are two possibilities. It may trigger the release of amyloid, the brain’s method of trying to contain an infection, and this may then mistakenly kill neurons. Or P. Gingivalis may directly damage the brain. We know that Alzheimer’s involves inflammation, an excessive immune response that ends up killing neurons instead of protecting them. P. Gingivalis is known to cause inflammation in gum tissue, and it may do so in the brain as well.
The speed at which damage accumulates is a key factor in the disease. Although many people harbor P. Gingivalis in their mouths, only some develop Alzheimer’s. Because it can be decades before Alzheimer’s symptoms appear, whether a person develops the condition could come down to how much damage occurs before they die of other causes.
Cortexyme reported in October 2018 that the best of its Gingivalis blockers, COR388, had passed the Phase 1 initial safety tests in people and had successfully entered the brain. It appeared to improve symptoms in participants with Alzheimer’s, but that was not part of the initial study. Cortexyme will launch a much larger Phase 2 clinical trial of COR388 later in 2019. Top
Brain Derived Neurotrophic Factor (BDNF Gene and BDNF Protein)
“Researchers at the Rush Alzheimer’s Disease Center in Chicago, Illinois reported intriguing evidence that people with higher levels of a "nerve growth factor" called BDNF tended to keep more of their cognitive functions even as amyloids built up. In fact people who had more BDNF activity saw 50% slower rate of cognitive decline over the study’s six years than those with lower BDNF activity” (Time Magazine, Feb. 2016).
The BDNF "gene" provides instructions for making a protein found in the brain and spinal cord called brain-derived neurotrophic factor, also called BDNF for short. Although related, one BDNF is a "gene" and the other BDNF is a "protein". The protein promotes the survival of nerve cells (neurons) by playing a role in the growth, maturation, and maintenance of the nerve cells. As a neuro-protein, BDNF is responsible for growing new neurons and repairing broken ones.
In the brain, the BDNF protein is active at the connections between nerve cells (synapses), where cell-to-cell communication occurs. The synapses change and adapt over time in response to human experience, a characteristic called "synaptic plasticity". The BDNF protein helps regulate synaptic plasticity, which is very important for learning and memory.
"Because BDNF plays a critical role in neuronal survival, synaptic plasticity, and memory, BDNF reduction may contribute to synaptic and cellular loss and memory deficits characteristic of Alzheimer’s Disease (AD). BDNF increases in the early stages of AD, which might reflect compensatory repair; however, it decreases as the disease progresses" (Wikipedia).
The BDNF protein is found in regions of the brain that control eating, drinking, and body weight; the protein likely contributes to the management of these functions. With a growing body of scientific evidence, it is no wonder that BDNF is quickly becoming one of the hottest compounds in brain health.
For decades, scientists have pursued an Alzheimer's cure with a single minded focus on how to treat the disease - get rid of the hallmark feature of Alzheimer’s which is the sticky protein plaques of amyloid (this has been very successful in mice). However, since 2000 more than 200 Alzheimer’s drugs have been tested, and none has proved to be a winner. Several drug companies have stopped trying and have cancelled all research projects. The situation is further confused by the fact that perfectly normal people also have some amyloid and tau plaque build up. Are the plaques really the enemy? Maybe BDNF is a solution - promote healthy nerve cells and live with the plaques.
Exercise is the most commonly known way of increasing BDNF, but people have also started looking towards other ways that they can increase this key protein for brain health. Unfortunately, many of the supplements that are touted for having the ability to increase BDNF, come up short due to the lack of research in humans (mice are frequently studied, but many results do not apply to humans).
Here are 4 supplements that "may" increase BNDF levels in your body.
1. Fish oil -There are several studies in animals that show when researchers induce dramatic brain injuries, the addition of omega-3 fats (like those found in fish oil) help to normalize levels of BDNF in these animals. Unfortunately, there are no human studies showing that taking a fish oil supplement will increase BDNF levels.
2. Curcumin - Curcumin, an extract from turmeric, is known for its anti-inflammatory properties, but some animal research suggests that curcumin can play a role in increasing BDNF levels. In one study, researchers took brain cells from mice and treated them with curcumin. This treatment with curcumin led to increases in BDNF levels. Unfortunately, there are no human studies showing that supplementing with curcumin will increase levels of BDNF.
3. Cocoa - Cocoa is known to be a powerful antioxidant. Research from Sbarro Institute for Cancer Research and Molecular Medicine, and the Center for Biotechnology at Temple University shows that cells that behaved as if they were inflicted with Alzheimer's disease were treated with antioxidants from cocoa. They found that the cocoa antioxidants turned on the cell's protective mechanism by activating the BDNF survival pathway. This research is very interesting, however, it is still a stretch to say that consuming cocoa will lead to increases in BDNF.
4. Coffee Fruit (NeuroFactor™) - The fruit from a coffee plant contains a powerful blend of antioxidants. The concentrated form of these antioxidants, called NeuroFactor™, has been shown - in two separate clinical studies - to increase BDNF levels in humans in less than 2 hours. This is the only supplement which has been clinically shown to increase BDNF levels in humans.
As you can see from the first three supplements above, there may be a plausible cellular mechanism for some of them to increase BDNF, but their ability to do so in humans has yet to be proven. Top
Balancing Glutamate And GABA
Glutamate and GABA are important neurotransmitters that have a major impact on our physical, mental and spiritual health. Neurotransmitters are chemical messengers that transmit information across a space junction mostly between ends of nerve fibers, but also muscle fibers. (Electrical messages cannot cross a space gap.) There are 6 major neurotransmitters. GABA and glutamate are 2 of the 6.
Glutamate is an excitatory transmitter and GABA is an inhibitor. Excitatory neurotransmitters stimulate brain cells, while inhibitory ones reduce stimulation. As in all neurotransmitters, too much or too little of either leads to problems. When both are working effectively, they keep each other in balance.
Glutamate is a primary excitatory neurotransmitter. It has many important roles like stimulating your brain cells so you can pay attention, talk, think, process and store information in short and long term memory. Studies suggest that the more glutamate receptors you have, the more intelligent you are. High levels of glutamate receptors are correlated with superior abilities in learning and memory. Unfortunately, they are also correlated with an increased risk of stroke and seizures.
Although glutamate is one of the most abundant neurotransmitters found in the brain, it exists in very small concentrations in any one location. If the concentration level rises, then neurons become too excited and don’t fire in a normal manner. When in excess, glutamate overstimulates brain cells and nerves which results in neurological inflammation and cell death. An excess of glutamate is a primary contributing factor to a wide variety of neurological disorders like Alzheimer's, Parkinson’s, schizophrenia, migraines, restless leg syndrome, fibromyalgia, multiple sclerosis and seizures.
GABA, which is short for gamma-aminobutyric acid, is the primary inhibitory neurotransmitter. Its primary role is to calm the brain, slow things down and relax you. GABA puts the pause between words when you speak. Without adequate GABA production, our conversations would consist of lots of run-on sentences, slurred speech, and we would have trouble comprehending language. Your gastrointestinal tract is packed with GABA receptors and it is critical for contraction of the bowel. Insufficient levels can result in abdominal pain and constipation. Insufficient levels of GABA result in nervousness, anxiety and panic disorders, aggressive behavior, decreased eye contact and anti-social behavior.
When GABA is low, glutamate is high and vice versa. Both are equally important. You might think of glutamate as the accelerator and GABA as the brakes. The goal is to achieve balance between the two.
The amino acid "taurine" increases GABA levels. Additionally, taurine doubles as an inhibitory neurotransmitter and can bind directly to GABA receptors, so it can help improve the GABA balance naturally. Taurine is found in high levels in the brain and cardiac tissue, indicating its importance in these areas. Taurine is found most abundantly in seafood and animal protein, so it is often deficient in one’s diet. Taurine is readily available as a supplement. It is cheap - about 10 cents a pill, and is highly recommended in a person's daily diet.
In order to increase GABA, one must eat a healthy diet (see the recommendations below), manage stress, avoid environmental toxins, support a healthy gut, get adequate sleep, and supplement with taurine. Top
"Reversing" Alzheimer's Disease

In 2014 researchers from UCLA and the Buck Institute for Research on Aging, led by Dr. Dale Bredesen, announced the results of a "first ever" study in which nine out of ten subjects diagnosed with dementia saw "reversals" of memory loss.
The approach used in the UCLA/Buck Institute study, called MEND (Metabolic Enhancement for Neural Degeneration), was personalized to each patient based on extensive testing to determine what was affecting the brain’s signaling network.
The one patient that failed to show improvement was unable to comply with the key components of the protocol. Nine out of the ten people in the study who did comply showed "meaningful reversals" in actual measurements of memory loss. However, the dietary/lifestyle changes were so difficult that none of the 10 participants were able to stick to the entire protocol.
An obvious criticism of the initial 2014 "MEND Study" was the small number of patients involved - only 10. Therefore, a paper in 2018 was completed on 100 patients, who were treated by several different physicians, which resulted in all 100 showing "documented" improvements in patient's cognition. The paper, REversal of COgnitive DEcline (ReCODE) with Dr. Bredesen as the primary author, was published in October, 2018 in the Journal of Alzheimer’s Disease. A PDF version of the paper can be "downloaded" by clicking here. ReCODE is Dr. Bredesen's updated version of MEND, the original reversal program.
The number of original patients in the new multi-physician test is unknown. It is believed that the second paper, detailing 100 successful patients, was written to show that the original nine "reversed patients" program was not a fluke. (To figure out the actual "reversal rate of all patients" (not just selected patients) would require an FDA type of program with very strict controls. More about that below.) Dr, Bredesen believes that by now (early 2019) there are over three thousand patients on the ReCODE protocol across the United States. A description of his "Ketoflex 12/3" diet (a major part of the ReCODE program) can be found here.
Alzheimer’s disease is the third leading cause of death of people over 65 in the United States, and the development of an effective treatment and prevention program is a major healthcare goal. However, clinical trials of drug candidates for Alzheimer’s disease treatment have been uniformly unsuccessful (more than 200 Alzheimer’s drugs have been tested, and there have been no winners).
There probably are several reasons for the repeated failures:
Given the long pre-period of no Alzheimer's symptoms, treatment is typically initiated very late in the process.
- What is normally referred to as Alzheimer’s disease is not a single disease, but one composed of several different subtypes (like cancer).
Just like other complex chronic illnesses such as cardiovascular disease, there are many potential contributors to Alzheimer’s disease, such as inflammation, various chronic pathogens, insulin resistance, vascular compromise, trauma, and exposure to specific toxins. Therefore, a mono-therapeutic approach is likely to be a failure. Personalized individual programs based on each patient’s genetics and biochemistry is the proper way to go.
- The model of Alzheimer’s as a single drug target (e.g., amyloid-β peptide plaques) is probably an inaccurate or incomplete model of the disease.
In the recent ReCODE test, the patients with cognitive decline were treated with multiple approaches and precision medicines. For those evaluated by the Montreal Cognitive Assessment (MoCA) or other similar tests, 72 of the 100 patients, there was a mean improvement of 4.9 points, with a standard deviation of 2.6. (MoCA scores range between 0 and 30. A score of 26 or over is considered to be normal. In a recent study, normal people scored an average of 27.4; people with mild cognitive impairment (MCI) scored an average of 22.1; and people with Alzheimer's disease scored an average of 16.2.)
Looking To The Future

The 100 person results should provide an adequate background to support randomized, controlled, clinical trials (as per the FDA). However, gaining approval for such trials is difficult since the trials will necessarily be multi-variable and non-uniform (i.e. personalized).
Dr. Bredesen's ReCODE program has not been clinically tested in large studies, such as those normally approved by the FDA, because it is seen as "too complicated". Most medical studies are designed to test a single drug in isolation. Dr. Bredesen has not been unable to have his protocol FDA tested because it does not conform to the standard testing method of a "single drug" or procedure against a "control group".
Developing a new drug takes years, if not decades, and costs an estimated $2.5 billion. Dr. Bredesen acknowledges that more research is needed. Therefore, a partnership program is currently underway with the Cleveland Clinic to initiate a much larger clinical trial of ReCODE.
The ReCODE program involves multiple strategies to address specific health issues that contribute to Alzheimer's Disease. The results of each strategy are measured by using blood tests, cognitive evaluations, and other markers of overall health improvements. Actions are tweaked over time to aim for optimal lab and evaluation results. The protocol is customized based on the patient’s genetics, current health, and lifestyle.
Much more on the Bredesen ReCODE program can be found here. Top
Summary Of Alzheimer's Recommendations
If you have had any family history of dementia it is recommend that you do the following:
-
Have your genes checked for either one or two APO E4s (click here). If positive - continue with the recommendations below. If negative, be aware of AD signals as a small fraction of people with no APO E4 genes do come down with dementia for unknown reasons.
-
Check how insulin resistant you are with an A1C test arranged by your family doctor. Your A1C should be less than 5.7. If 5.7 or more, reduce your total carbohydrate intake to less than 40 grams per day until your A1C is less than 5.7.
-
Exercise for a minimum of an hour a day, 5 or 6 days a week. Exercise is key to improving memory as it increases the blood flow of oxygen and other key ingredients to your brain (and other organs).
-
Keep your teeth and gums clean and free of plaques. Ninety plus percent of Alzheimer's victims have dental Gingivalis in their brains. Brush and floss your teeth twice-a-day along with a twice-a-day mouth bacteria killer rinse such as Scope.
-
Fast 14 hours a day, say from 7 pm to 9 am. This allows ketones to cleanse damaged proteins and mitochondria (cell energy producers) from your brain and promote the growth of neurons. When your body runs out of energy from food, your liver produces ketones from body fat. This is called mild ketosis (starvation is full ketosis). Mild ketosis as well as exercise increases the production of BDNF, which promotes brain plasticity and memory (see the BDNF section above).
- Add a “taurine” supplement to your daily diet to increase your GABA balance.
-
Follow one of the two Alzheimer's oriented diets - The MIND Diet (here) or the Ketoflex 12/3 Diet (here). Both have good records as far as reducing the probability of Alzheimer's and are quite similar.
-
Read Dr. Bredesen's Book "The End Of Alzheimer's" and follow the ReCODE Program he has designed for Alzheimer's patients, especially those with mild Mild Cognitive Impairment (MCI).
-
Brief yourself on the "APO E4 Web Site" (https://www.apoe4.info/wp/welcome/) and follow their recommendations.